The drug development landscape is notoriously grueling, characterized by extensive research periods and staggering financial costs. For every promising candidate that makes it into clinical trials, there exists a haunting statistic: over 90% fail to make it past this pivotal stage. The reasons for such dismal outcomes are multifaceted, with safety concerns topping the list. Traditional methods of assessing a drug’s safety often involve a lengthy, trial-and-error process, which not only consumes resources but also places patients at risk. As the world demands quicker and safer drug formulations, the need for innovative solutions has never been greater.
AI Models: A Game-Changer in Drug Safety
Researchers at the Broad Institute of MIT and Harvard have taken a bold step towards transforming this landscape by harnessing the power of artificial intelligence. Their exploration into the predictive capabilities of AI for drug toxicity aims not just to enhance efficiency but to redefine the foundational processes of drug development. Led by Srijit Seal, a visiting scholar at the Carpenter-Singh Lab, this initiative has devised multiple machine learning models that assess the potential biological impacts of drugs prior to human trials. By analyzing a drug’s chemical and structural characteristics, these tools effectively forecast safety risks related to cellular health, pharmacokinetics, and organ functions like the heart and liver.
Bridging the Gap Between Data and Toxicology
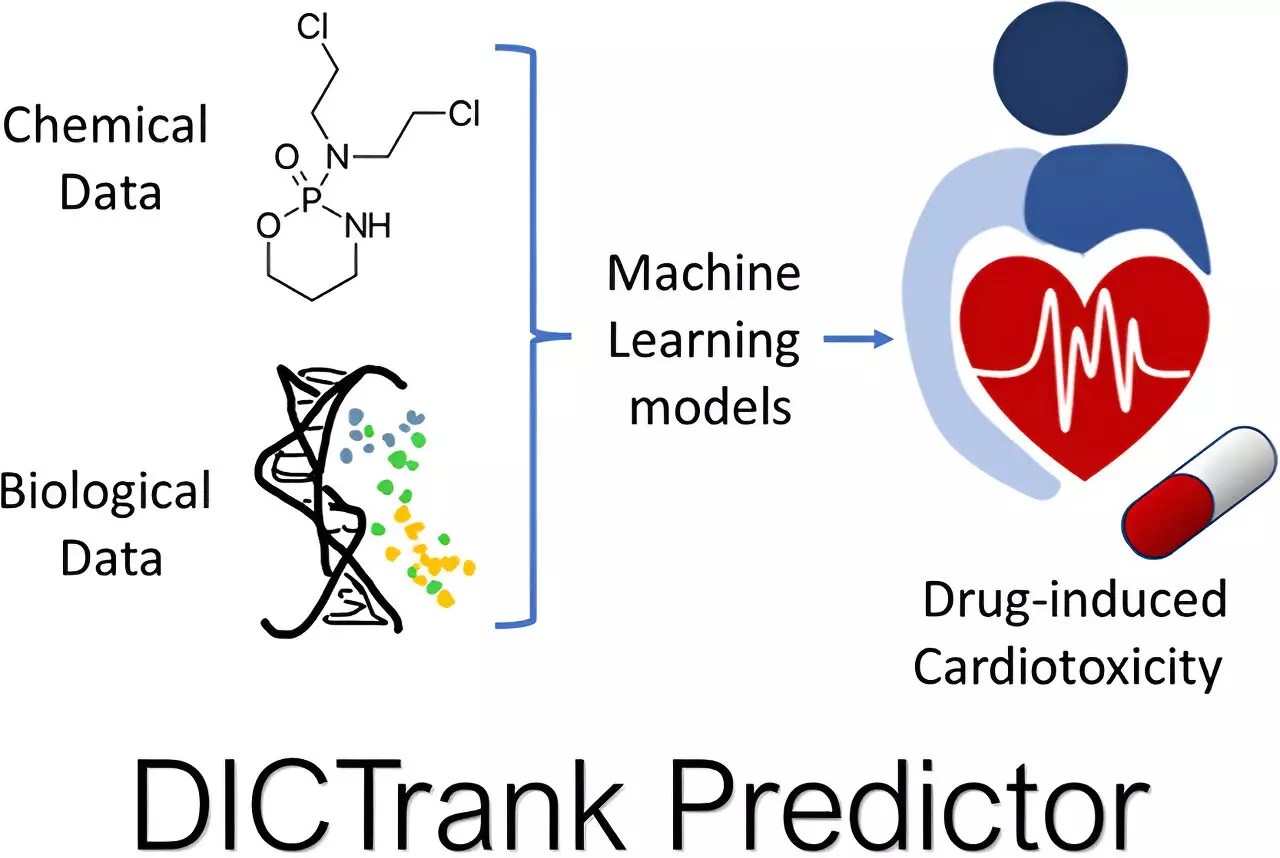
While traditional toxicology methods have been historically reliant on laboratory experiments, the introduction of these predictive models serves to complement rather than replace existing practices. Seal’s motivation to delve deeper into drug toxicity stemmed from a pressing question often overlooked: Can the chemical structure of a candidate predict its toxicological profile? This query led to the development of two specific models targeting cardiotoxicity and liver injury, utilizing extensive datasets curated by the FDA. By integrating these databases with relevant physicochemical properties, the models reveal intricate links between chemical features and toxicity—an area ripe for exploration and innovation.
The implications are significant. Both DICTrank Predictor and DILIPredictor exemplify this innovative approach; while the former anchors itself as the first model aligned with the FDA’s cardiotoxicity ranking, the latter navigates the complexities of differentiating between human and animal toxicity. This dual capability reveals a profound understanding of the biochemical drivers behind toxicity and underscores the necessity for early-stage predictions.
Pharmacokinetics: A Critical Consideration
An equally paramount concern in drug development is pharmacokinetics—the study of how a drug is absorbed, distributed, metabolized, and excreted in the body. Timely insights into these pharmacokinetic parameters are essential; drugs that fail to reach their intended targets risk ineffectiveness, while those that linger in the body may provoke adverse reactions. Seal’s ongoing collaboration has introduced a predictive pharmacokinetic modeling tool that could not only streamline this process but also instill a “fail faster” philosophy within research teams. This rapid feedback mechanism promises to save valuable time and resources, enabling developers to focus on candidates with robust bioavailability profiles.
Cell Health Insights Through Advanced Imaging
Beyond predicting toxicity and pharmacokinetics, it’s crucial to understand the cellular ramifications of drug compounds. Seal’s exploration has led him to develop BioMorph, an advanced deep learning model that synthesizes data from CellProfiler—an open-source software known for its capability to assess cellular characteristics through image-based information. The innovation does not stop at merely analyzing these images; BioMorph enhances the biological interpretability of cellular responses to compounds. This not only aids researchers in deciphering the complex interactions at play but also opens new avenues for understanding how specific compounds might alter cell health.
The combination of AI-driven analysis with practical lab methodologies represents a leap forward in pharmacological research, one that promises a more nuanced understanding of drug effects at the cellular level. As such, BioMorph can guide scientists in predicting not just the existence of toxicity but also its underlying biological mechanisms.
The Future of Drug Development: A Transformative Shift
The initiatives being spearheaded at the Broad Institute could redefine the drug discovery paradigm. By marrying traditional research methods with advanced machine learning, the outlook for early-stage drug development becomes both safer and more efficient. The potential to reduce failure rates and expedite testing not only has ramifications for the pharmaceutical industry but could also enhance patient safety and accessibility to therapeutic options. As AI technology continues to evolve, the intersection of computational analysis and biological experimentation will likely yield profound innovations in how drugs are designed and assessed. The quest for better, safer drugs accelerates, and with it, the promise of a healthier future for all.

Leave a Reply